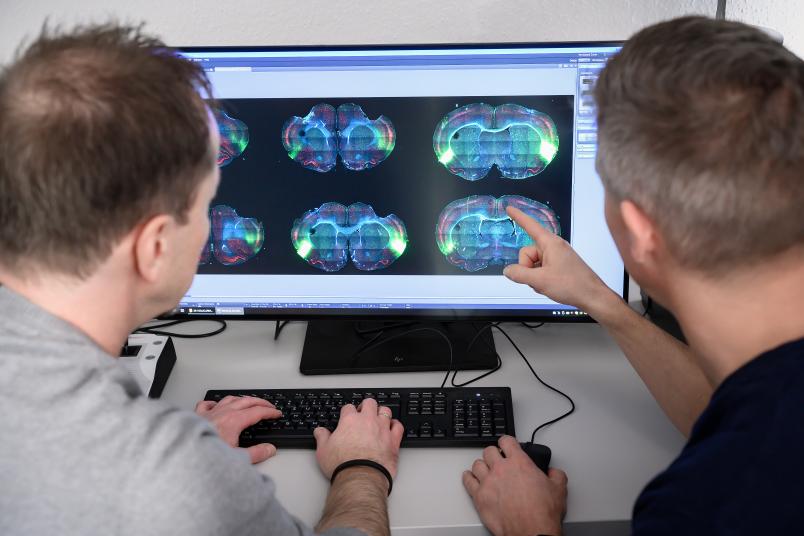
Immune system
The Sixth Sense
Immune responses can affect our mood and interfere with learning. Conversely, learning processes can influence immune responses.
The nose is running, the next pack of tissues is empty, and there is that nagging cough. As if that wasn’t bad enough, your head is pounding and you can’t get anything done. Lack of appetite, fatigue and a bad mood perfectly round out the feeling of having an infection. Surely, everyone has experienced situations like this. When we feel physically ill, this also depresses our mood.
“That sounds negative, but it’s actually an adaptive response by our body to protect us and others: You take it easy, avoid contact and stay at home,” explains Professor Harald Engler. He is an expert in behavioral immunobiology at University Hospital Essen, which is part of the University of Duisburg-Essen and cooperates with Ruhr University Bochum within the University Alliance Ruhr.
Immune system causes a drop in mood
Engler is interested in how the immune system affects the nervous system. “Our immune system serves as our sixth sense,” he says. “It detects things that we are unable to see, hear or smell because they are too small – such as pathogens and inflammatory processes – but which nevertheless pose a threat to our body.” If the immune system impacts on our mood and behavior, it is a protective mechanism. “Things get problematic when people suffer from chronic inflammation, as in the case of arthritis or inflammatory bowel disease,” says Engler. “As a result of this, they can develop clinical depression, the cause of which does not primarily lie in brain metabolism but in inflammation.”

Somewhere a switch gets flipped. Then the immune mechanism that affects our mood and behavior is no longer adaptive, but harmful.
As part of the Collaborative Research Center on Extinction Learning, which is coordinated at Ruhr University Bochum, Engler and his colleagues are investigating when the protective mechanism becomes a problem. “Somewhere a switch gets flipped. Then the immune mechanism that affects our mood and behavior is no longer adaptive, but harmful”, he explains. The relationships are complex. This is why Engler’s work covers the entire spectrum from animal experiments to studies in healthy people and patients. From the many pieces of the puzzle, the researchers hope to one day gain a better understanding of the interactions between inflammation, learning processes, and the mind.

Extinction learning seems to play an important role. The term is related to classical conditioning, which became famous through Pavlov’s dog experiments. Pavlov repeatedly combined a neutral stimulus, the ringing of a bell, with a second stimulus: the provision of food. Over time, the dog learned that the bell signaled the presentation of food, and responded solely to the sound by salivating. However, when the presentation of food stops, the connection between the bell and the food is unlearned, a process called extinction.
Disrupted extinction learning could promote illnesses
Harald Engler’s team is pursuing the hypothesis that some disorders are associated with altered extinction learning and that inflammatory processes can disrupt successful extinction. Together with the team of Bochum researcher Professor Sigrid Elsenbruch, his group recently demonstrated such an effect in healthy volunteers. The study participants first learned to associate geometric symbols with pain. They repeatedly saw images of triangles, circles and squares, for example. Some of these symbols, e.g., the triangle, were paired with a brief pain stimulus. Thus, after the learning phase, the subjects rated the triangle more unpleasant than the other geometric symbols. On the following day, extinction learning took place: Now none of the geometric symbols was associated with pain. After the extinction phase, the participants had to rate again how unpleasant they perceived the different symbols. At the same time, the researchers recorded their brain activity with functional magnetic resonance imaging.
Inflammation slows down extinction learning
The results were dependent on whether or not the subjects had experienced an inflammatory response during the learning on the first day. “By administering a very low dose of a bacterial component, we can safely induce an inflammatory response along with depressive mood in healthy people over a period of several hours,” explains Harald Engler. “After 24 hours, the effects are completely gone.”
If the participants learned the association between the geometric symbol and the pain stimulus under the influence of this experimental inflammation, the researchers observed increased neural activation in what is known as the brain’s fear network when the geometric symbol was presented during extinction learning. In addition, the test subjects also rated unannounced pain stimuli as much more unpleasant than people who had only been given a placebo on the first day. This indicates that inflammatory processes strengthen the memory trace for pain-associated stimuli.
“We assume that this mechanism could foster the development of chronic pain,” concludes Harald Engler. The researchers now want to further investigate this theory and the underlying mechanisms in an animal model and in patients with chronic inflammatory disease.

The brain and immune system communicate through complex bidirectional pathways.
While Harald Engler is trying to promote extinction learning and to erase pain associations, Professor Martin Hadamitzky’s research aims towards the opposite direction: He also works in the field of behavioral immunobiology at University Hospital Essen, but he wants to prevent the extinction of learned associations. Hadamitzky investigates how learning processes influence immune responses. “The brain and immune system communicate through complex bidirectional pathways,” emphasizes Hadamitzky.
Sugar water instead of medication
In animal studies, Hadamitzky’s team pairs neutral stimuli such as sugar water with the administration of an immunomodulatory drug. For example, the researchers are working with rapamycin, a compound which inhibits the growth of tumors. They showed that rats were able to learn an association between the taste of the sugar water and the immunological changes induced by rapamycin: If the animals repeatedly received a combination of sugar water and the drug, the administration of sugar water alone ultimately led to substantial effects on the rats’ immune system.
“However, these effects eventually disappear at some point due to extinction learning,” says Hadamitzky. Together with his colleagues, he is thus looking for ways to prevent conditioned responses from being extinguished or relearned. “Our idea is to give the immune system a reminder,” explains the neurobiologist. For the initial learning phase in the conditioning experiments, the researchers administer a clinically relevant dose of rapamycin, i.e. an amount of active ingredient that has been shown to efficiently prevent tumor growth. After the animals have learned the association between the sugar water and the immunological action of rapamycin, the researchers did not stop administering the drug completely, but continued to pair the sugar water with only ten percent of the original drug dose.
Reminding the immune system of what has been learned
“This is a subeffective dose,” explains Martin Hadamitzky. “If we only work with this ten percent dose right from the start, it has no effect on tumor growth at all.” Things look different if the animals have previously learned an association between sugar water and the full rapamycin dose. In this case, the ten percent dose is sufficient to attenuate tumor growth just as much as if animals were treated with the full dose and without associative learning. The subeffective dose thus acts like a reminder cue for the immune system and slows down or even prevents extinction learning.
In further studies, Martin Hadamitzky wants to find out what exactly happens in the brain. The underlying mechanisms have to be well understood in order to potentially transfer their findings to humans. After all, the researchers cannot simply experiment on patients whose survival depends on a drug. Following extensive animal studies, investigations are therefore initially planned with healthy volunteers and patients with less severe disease conditions, such as inflammatory skin reactions.
Increased quality of life for patients
However, Martin Hadamitzky and Harald Engler envision that their work will one day offer patients better quality of life. “Many people, such as cancer patients or patients who have received an organ transplant, have to take drugs with severe side effects on a daily basis,” explains Harald Engler. “It will never be possible to go without medication completely, that would be an illusion,” adds Martin Hadamitzky. “But if we could reduce the dose while maintaining the therapeutic efficacy, that would be a huge win.” As if it were possible to prevent harmless inflammation from triggering depression.