Computer Simulation
Heating Efficiently
Researchers from the Collaborative Research Center Bulk Reaction have set out to understand how the heat distribution in large-scale industrial furnaces affects the products inside. This should aid the energy transition.
Pills, coffee beans, limestone, wood chips and waste, to name but a few: Many of the products we use everyday have undergone thermal treatment. This means that they are heated in furnaces to be dried, chemically modified, roasted or incinerated. These furnaces can be quite huge. Their interior usually remains a black box, as the frequently very high temperatures, densely packed materials and in some cases aggressive atmospheres mean that it is extremely difficult to deploy measurement technology inside.
“We can draw on the experience of past decades,” says Professor Martin Schiemann from the Institute of Energy Plant Technology at Ruhr University Bochum. As long as the product quality and price were acceptable, the process could remain as it had always been. But those days are over: Energy is in short supply and very expensive, and we want to move away from fossil fuels such as natural gas. That’s why the industry has begun to look at what’s going on inside the furnace and how it can be heated as efficiently as possible, possibly using either hydrogen or electricity.
Stones transfer radiant heat
As part of the Collaborative Research Center Bulk Reaction, Martin Schiemann and PhD student Matthias Tyslik are developing ways to simulate the processes that take place in such furnaces. Primarily, they want to find out how the heat is distributed, for example in lime kilns with a height of tens of meters. It can hold more than 100 tons of limestone, which is roughly crushed, poured in from above and removed from below. Each stone remains in the kiln for more than 24 hours. The kiln is heated by a lateral gas flame that rises to the top. The flame itself reaches temperatures of around 1,400 degrees Celsius, while the opposite side of the kiln has to be heated to at least 850 degrees Celsius. The heat treatment is intended to trigger a chemical reaction in the limestone. As a result, calcium carbonate is to be converted into calcium oxide, removing CO2 from the stone. The aim is to ensure that all stones that are removed at the bottom of the kiln have undergone a complete reaction.
SFB Bulk Reaction
“This is why we need to know exactly how the heat propagates in an industrial furnace,” explains Matthias Tyslik. The heat is transferred from the surface of one stone to the next by radiant heat, for example. This process depends on the local conditions: The transfer can only take place by visual contact, not across obstacles. The temperature difference between the individual stones is also important.
Calculations within reasonable time frames
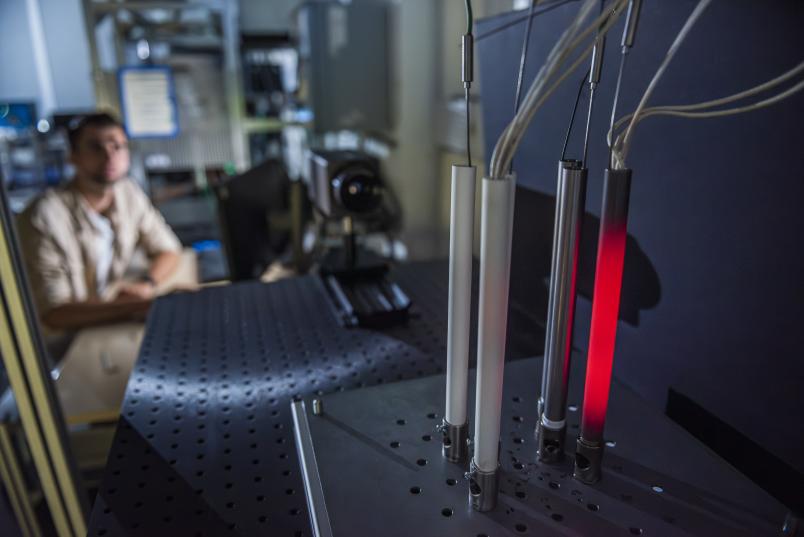
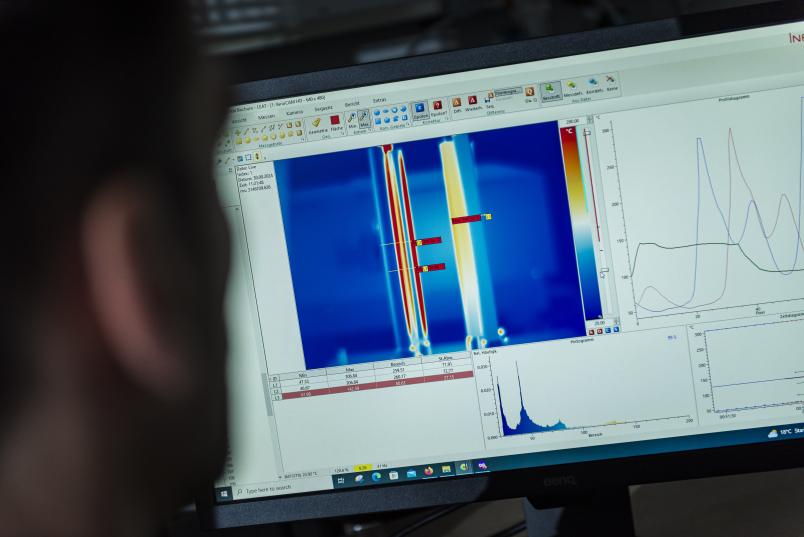
In order to analyze the details of the heat transfer, the researchers have set up a number of experiments in their lab. One of the experiments involves a thermal imaging camera observing an simplified bulk of stainless steel respectively magnesium rods, one of which is heated. The rods are much easier to calculate geometrically than crushed stones or fibrous wood pellets. This geometrically simple structure can thus help answer a number of questions: Does heat get reflected? Which rod heats up first? How relevant is the material? Other experiments focus on how heat transfer changes when the bulk material moves in the process.

“If you know all parameters, you can theoretically calculate such variables – even for the millions of stones in a lime kiln,” says Martin Schiemann. “But it’d take so much time and computing capacity that it would be practically impossible.” That’s why one of the goals is to simplify the simulation so much that it can be carried out in a reasonable time period without compromising on accuracy.
Even if a handful of coffee beans gets too hot, you can no longer use the whole batch.
It would then be possibleto calculate, for example, how the thermal treatment of the limestone would have to be modified if natural gas – which is commonly used today – would be replaced by hydrogen as a fuel. “You can’t simply swap them, because hydrogen combustion has a completely different combustion process,” explains Martin Schiemann. “The flame would probably be shorter, and the process would produce entirely different pollutants that we’d then have to deal with.” In addition to hydrogen, ammonia could also be used as fuel for lime kilns “So far, it is been very difficult to heat them using electricity in order to reach the necessary temperatures of over 1,000 degrees in such dimensions,” points out the researcher.
However, it might certainly be feasible for other processes. Pills, for example, are dried at a maximum of 100 degrees Celsius, while coffee beans are roasted at up to 300 degrees Celsius. Again, the distribution of heat in the furnace is crucial. “If even just a handful of beans get too much heat, you can no longer use the whole batch,” points out Matthias Tyslik.

Other processes present a different set of challenges. Unlike in the lime kiln, for example, the components that make up the bulk material in waste incineration are extremely heterogeneous. “There are sometimes mattresses in there, or PET bottles that melt into little lumps when heated,” explains Martin Schiemann. The aim of the Collaborative Research Center is to develop a simulation method that can be adapted to all of these processes. Other sub-projects explore, for example, the path that individual particles take in a furnace, the gas flow in the furnace and heat transfer through direct contact between individual particles.