
The researchers developed the transparent cylinders and small Teflon balls themselves for the experiments.
Photochemistry
Shaken, not Stirred
The correct movement facilitates light-driven reactions without the use of environmentally harmful solvents.
Chemical reactions require energy to be initiated. Using light as an energy source can provide a sustainable approach: It is abundant and environmentally-friendly. “A major disadvantage of photochemical reactions is that they usually occur in solution and require high dilution,” says Carolina Spula. Since solvents make up the majority of chemical waste, Spula avoids their use. She works with Professor Lars Borchardt on solid-state photochemistry in ball mills at the Chair of Inorganic Chemistry I. The reactions take place in the dry state-without solvents and driven by light. Nonetheless, certain challenges remain.
“Conventional solid-state photochemistry is limited to very small quantities,” explains Spula. Because light cannot deeply penetrate into solid powders, the conventional method used very thin powder layers spread onto a glass plate and illuminated it from below. This raised the question: How can larger quantities of powder be uniformly exposed to light to enable reactions throughout the whole mixture?

Carolina Spula is completing her doctoral thesis in the Mechanochemistry working group.
To achieve this, Carolina Spula employs movement. In her doctoral research, she developed specialized photoreactors for ball mills to allow irradiation of the reaction vessels, while shaking. These consist of a transparent quartz glass cylinder containing several small Teflon balls. The powdery starting material for the desired light-driven reaction is poured into the vessel and sealed with polymer caps on both ends. The tube is mounted into a ball mill that shakes it horizontally while illuminating it uniformly from all sides by several UV lamps.
One third powder, one third balls, one third air
During her research, Spula refines this setup. “The general rule of thumb is that a successful reaction requires one third of powder (by vessel volume), one third balls, and one third air,” she explains. “For some reactions, it may be helpful to add a small amount of bulking agent, for example an inert salt. This prevents the powder from clumping together in a corner where it would no longer be exposed to light.” The bulking agent can later be separated. Sometimes, also small amounts of solvent are used to create a paste-like consistency within the tube.
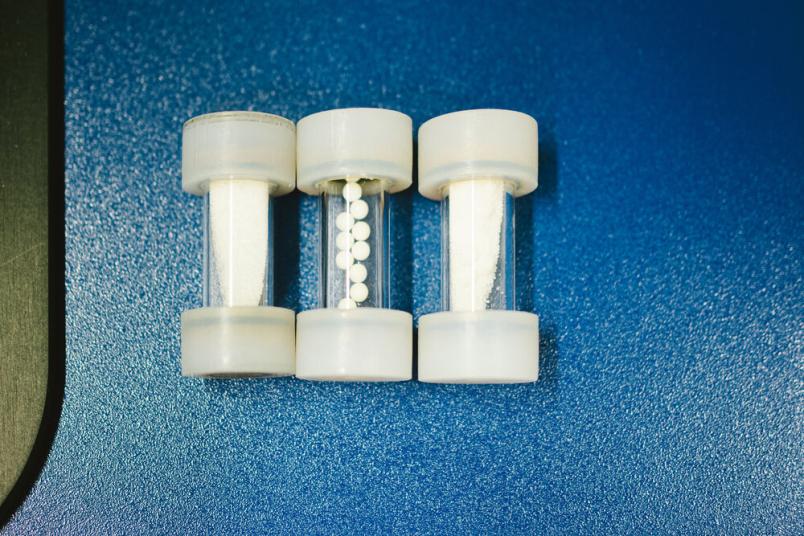
The rule of thumb is: one-third powder, one-third pellets, one-third air. If you don't use pellets, you can add more powder.
To realize photochemical reactions for larger powder quantities Spula tested an alternative instrument with a different motion-pattern: the resonant acoustic mixer. In this approach, the vessel is mounted upright and set it into vertical vibration. “In some ways, it can be compared to the paint mixers you find in hardware stores,” she explains. The vertical vibrational oscillation generates a main mixing stream accompanied by smaller turbulences, ensuring thorough mixing. As a result, every powder particle reaches the transparent outer-wall of the vessel at some point where it is exposed to light. In this setup, no milling balls are required, allowing for greater powder loading.
Spula is working on organic reactions. One example is the cyclodehydrochlorination. In this reaction, a chlorine atom is removed from a chlorinated precursor consisting of a carbon-based chain system. The ring system closes at the exact position of the carbon-chlorine bond breakage. Since the bond between the chlorine and the carbon is significantly weaker than the bond between two carbon atoms, the light particles (photons) excite the electrons responsible for the bond, selectively breaking the C-Cl bond and no other while leaving the carbon framework intact. The carbon ring then closes precisely at exactly the designated position.
Nanographenes could replace silicon
Utilizing this reaction, Spula synthesized nanographenes, which may serve as an alternative material in microelectronics replacing silicon. “The goal is to obtain long planar carbon chains,” Spula explains. Because of their adjustable band gap, such graphene nanoribbons have the potential to replace silicon semiconductors in microelectronics one day, whose band gap is limited. Graphene nanoribbons could increase this limit.
For nanographenes a precise geometry is crucial: The structure must be planar. To prevent ring closure on the reverse side due to twisting of the precursor molecule, the chlorine atom is incorporated as a kind of predetermined breaking point.

Irradiated from all sides with special UV light, the reagents are shaken horizontally in the grinding beakers together with small balls.
Although such reactions are also possible in solution, the solubility of larger carbon chain precursors, decreases sharply with the size. “Researchers then rely on harsh solvents that are harmful to the environment to overcome this problem,” says Spula.
The solvent-free solid-state photochemical synthesis of nanographene avoids this problem. “For example, it took us 25 hours in the mill to convert 150 milligrams of a terphenyl ring,” she says. “The reaction of a double-chlorinated pentaphenyl system required 48 hours.” The chemist used analysis methods such as nuclear magnetic resonance spectroscopy to verify the product formation.
“So far, we have only performed this work on a laboratory scale,” Spula explains. “This allowed us to demonstrate that the method works in principle.” Beyond nanographenes, the approach may also be valuable in broader areas of organic synthesis, including the production of fertilizers or pharmaceutically active compounds without the use of metal-based catalysts.
Applicability with industrially significant bonds
In her dissertation, she addressed the question of whether the method is limited to ring-closure reactions or can also be used for the general functionalization of carbon-halogen (C-X) bonds where X can be chlorine, bromine, or iodine. “Conventional C-X functionalizations usually require transition metals and hazardous reagents, which results in high costs, waste generation, and pollution,” says Spula. “Photochemical alternatives are known, but they typically require highly diluted solutions.”
She developed a metal- and catalyst-free synthesis protocol for photochemical bond formation between carbon and boron to produce arylboronic acid esters, an important class of molecules in organic synthesis, sensor technology, the manufacture of pharmaceuticals, and materials science. She has been able to expand the protocol to carbon-phosphorus and carbon-sulfur bonds. These molecule classes are used in pharmaceuticals and as fertilizers in agriculture, among other applications. “The evaluation has shown that our UV-driven mechanochemical method has the best evaluation in terms of green chemistry metrics with the lowest energy consumption compared to solvent-based or metal-catalyzed methods,” the researcher explains.