Biology
New insights into the function of a common signal-protein complex
From microorganisms to humans – all living organisms with a cell nucleus possess the Stripak signal-protein complex. A research team has gained new insights into its functions.
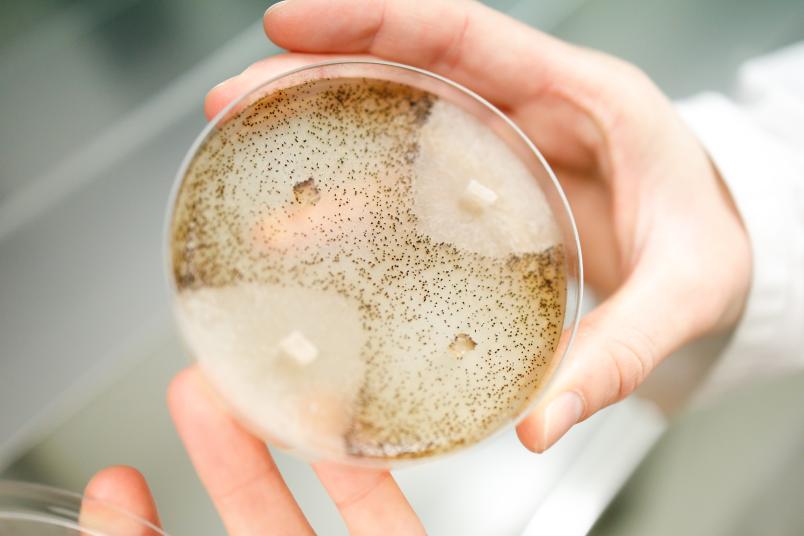
Researchers from Bochum, Düsseldorf and Dortmund have gained new insights into how the Stripak signal-protein complex works. They studied the complex, which occurs in all organisms with a cell nucleus, in the fungus Sordaria macrospora and showed with which target proteins it interacts. In humans, Stripak defects may lead to diabetes and heart attacks and in fungi to sterility. The current study shows that the complex is important for cellular transport processes. The team headed by Valentina Stein and Ramona Märker from the Department of General and Molecular Botany at Ruhr-Universität Bochum describes the results in the journal Plos Genetics from 30 September 2020.
Target proteins were not known
The Stripak protein complex fulfils a similar function in humans and in micro-organisms: it activates proteins specifically by attaching phosphate groups. However, it had not yet been understood which proteins exactly are modified by the complex. In February 2020, Bochum-based researchers Valentina Stein and Ramona Märker, in collaboration with Bernhard Blank-Landeshammer from ISAS Leibniz Institute in Dortmund, came a step closer towards answering this question. In a first, they identified target proteins that the Stripak complex specifically modifies, namely the protein Cla4, which is highly conserved in all living organisms and controls cell death or brain development in humans.
Crucial for vesicular transport
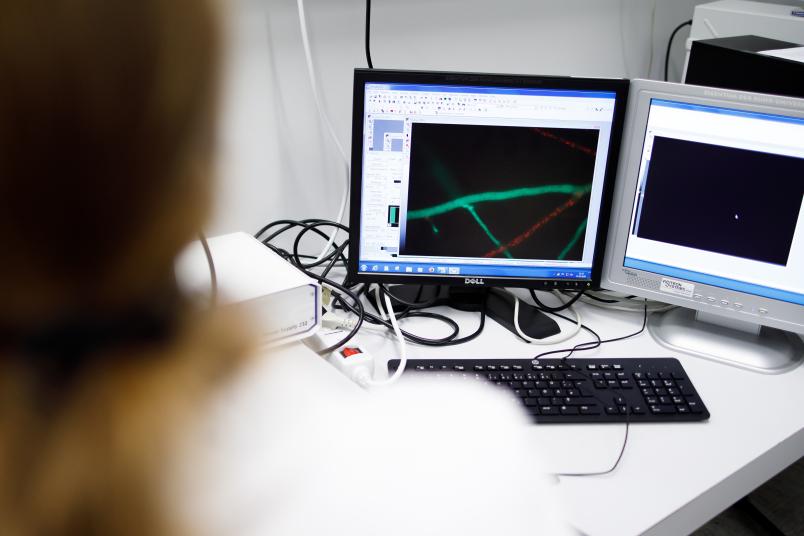
In a further study, Valentina Stein’s team now describes a new type of target protein of the Stripak complex. Together with Kira Müntjes from the Microbiology Department of the University of Düsseldorf, the researchers showed that the RNA-transporting protein GUL1 is a target protein of Stripak. GUL1 migrates on vesicles and is crucial for the directed transport of RNA in the cell. The vesicles, in turn, are important for cellular transport processes, for example to transport hormones or messenger substances. The group also showed that sexual development is disturbed in the analysed fungus if the target protein GUL1 is only incompletely modified with phosphate groups.
The team thus demonstrated for the first time that the ubiquitous Stripak complex also controls cellular vesicular transport.
“The Stripak components of fungi and humans are functionally highly conserved. Therefore, the findings are also relevant for application in medical research, as they might help to treat disorders in vesicular transport,” says Valentina Stein.