Eletrochemistry
Maximizing Hydrogen Peroxide Formation during Water Electrolysis
When water is split electrolytically, the result is typically hydrogen – and useless oxygen. Instead of oxygen, you can also produce a useful product. If you know how.
Due to its high availability, water is considered the most useful starting material for hydrogen production. Ideally, the conversion of water into hydrogen produces a second useful substance: hydrogen peroxide, which is required for many branches of industry, for example for the production of disinfectants. Special reaction conditions are required to obtain hydrogen peroxide from the splitting of water. It was known that the presence of carbonate is useful. But why this is the case was unclear. A team from Ruhr University Bochum, Germany, has now elucidated the mechanism behind this.
The group led by Dr. Lejing Li, Dr. Carla Santana Santos and Professor Wolfgang Schuhmann from the Center for Electrochemistry at Ruhr University Bochum describes the results in the journal “Angewandte Chemie International Edition” from 24 June 2024.
Killing two birds with one stone
“Hydrogen peroxide is a valuable chemical that has to be produced using complex processes that are not always harmless to the environment, says Wolfgang Schuhmann. It would be useful if the substance could be obtained in large quantities from the electrolytic splitting of water, which also produces the energy carrier hydrogen. “However, this is thermodynamically complicated," explains Lejing Li. This is because the production of oxygen is, so to speak, energetically simpler.
However, if a carbonate buffer is added to the solution, the situation changes. This is carbonic acid (H2CO3), which can release a proton (H+), resulting in hydrogen carbonate (HCO3-), which can react further to form carbon dioxide (CO2). Such buffers help to keep the pH value of solutions stable. However, the conditions in the reaction solution are not identical everywhere.
The conversion of water to hydrogen and oxygen takes place at the surfaces of two electrodes, between which a voltage is applied. When negatively charged electrons are transferred, positively charged protons are released at the same time. The protons change the pH value in the immediate vicinity of the electrode, while the pH value remains stable further away in the solution.
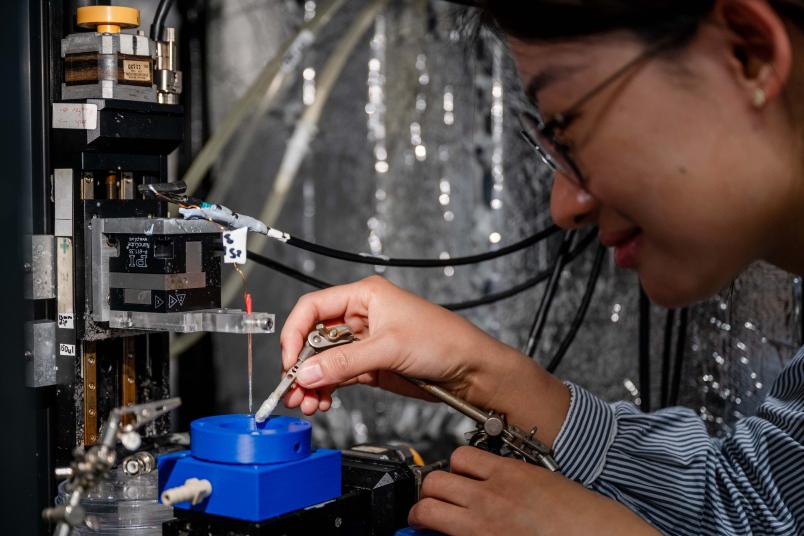
Local pH value measurements
Using a method they developed themselves, the Bochum team determined the pH value in the immediate vicinity of the electrode under different reaction conditions and showed that hydrogen peroxide is preferentially produced when there is a lot of hydrogen carbonate in the vicinity of the electrode. Under these conditions, an intermediate reaction product is formed that prevents the formation of unwanted oxygen.
“These results initially sound like abstract basic research,” admits Lejing Li. “But the production of hydrogen and hydrogen peroxide is extremely important. Only if we understand the processes precisely we can make them better.”