Chemistry
Local Solvation is Decisive for Fluorescence of Biosensors
Nanotubes can serve as biosensors. They change their fluorescence when they bind to certain molecules. Until now, it was unclear why. Researchers have gained new insights into the cause of the fluorescence.
Researchers from Bochum and Texas have discovered why carbon nanotubes fluoresce when they bind to certain molecules. Nanotubes are considered promising biosensors that could be useful for blood sugar monitoring or Covid-19 tests, for example. When they bind to an analyte, they fluoresce – the higher the concentration of the analyte, the brighter the fluorescence. Researchers from Ruhr University Bochum, Germany, and a team from the University of Texas at El Paso, USA, have used terahertz spectroscopy to unveil the mechanism behind the light emission. They showed that the aqueous solution plays a decisive role for the fluorescence. The results have been published online in the journal “Nature Communications” on August 8, 2024.
At Ruhr University, the groups of Professor Martina Havenith and Professor Sebastian Kruss collaborated for the study, which took place as part of the Cluster of Excellence “Ruhr Explores Solvation”, RESOLV for short. The PhD students Sanjana Nalige and Phillip Galonska made significant contributions.
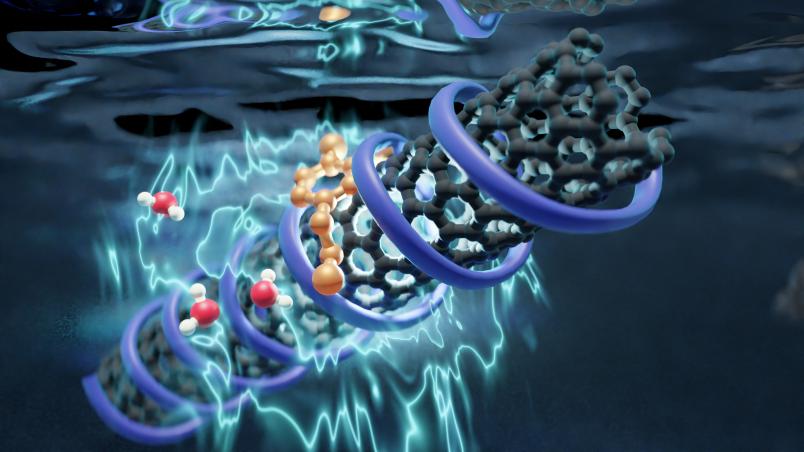
Carbon nanotubes as biosensors
Single-walled carbon nanotubes are powerful building blocks for biosensors, as previous studies revealed. Their surface can be chemically tailored with biopolymers or DNA fragments to interact specifically with a certain target molecule. When such molecules bind, the nanotubes change their emission in the near-infrared range, which penetrates deep into tissue. This way, for example, the presence of certain neurotransmitters, i.e. messenger substances in the brain, can be detected. Although such sensors are already in use, their exact functional principle has been unclear.
Water is decisive for fluorescence
Because most relevant biological processes take place in water, the researchers analyzed the carbon nanotubes in an aqueous solution. Using terahertz spectroscopy, they were able to detect how energy flows between the carbon nanotubes and water. The decisive factor is the hydration shell of the biosensors, i.e. the water molecules surrounding the nanotubes. When a carbon nanotube is excited, the internal energy can couple to the vibrations of the hydration shell. Energy flows between the water and the nanotubes: Sensors that become brighter in the presence of the analyte transfer less energy into the water. In contrast, sensors that become dimmer transfer more energy into the water.
“Terahertz spectroscopy allows us to measure directly what we had previously only suspected,” says Sebastian Kruss. “These insights provide a general and rational design principle to develop optimal biosensors with the best performance for novel applications in research and medicine.“
Martina Havenith, spokesperson of the Cluster of Excellence RESOLV, adds: “In this interdisciplinary study, we did not put the spotlight on the carbon nanotube itself. Instead we put the spotlight on the solvent, water, and discovered a previously unknown direct correlation with the changes in the water around the carbon nanotube and the function as a biosensor. This is exactly what RESOLV stands for.”