Medicine
How Aortic Valve Stenosis Develops
The endothelium lining the valve plays a critical role. It’s very important whether the valve consists of two or three leaflets.
There is currently no way to stop calcification of the aortic valve. If all else fails, the valve must be replaced. To better understand the development of this common disease, researchers from Bochum and Bonn have taken a close look at defective valves. They were able to show that endothelial cells that line the tissue play a major role, as is the case with other vascular diseases. In addition, they were able to see that what happens varies greatly depending on whether someone’s aortic valve has three leaflets – as is usually the case – or just two. The team reports its findings in the Journal of the American Heart Association on June 25, 2025.
Many people around the age of 60 experience a decline in physical performance due to aortic valve stenosis. In this condition the heart valve, which is located between the left ventricle and the aorta becomes calcified. This makes it harder to open, hindering the flow of blood from the heart to the body. “This disease is common; however, its development is still poorly understood,” explains Professor Daniela Wenzel, Head of the Department of Systems Physiology at the Faculty of Medicine at Ruhr University Bochum. “That’s why there is currently no way to stop it. Only when there is absolutely no other option the heart valve needs to be replaced.”
Wenzel and her colleagues, who are part of the Collaborative Research Center/Transregio 259 “Aortic Diseases,” want to get to the bottom of the pathogenesis of the disease. They are particularly looking at the endothelium, meaning the single layer of cells that surrounds the aortic valves. “Among other things, these cells ensure that platelets do not adhere and clots cannot form,” says Wenzel. “We know that the endothelium also plays a significant role in vascular diseases, such as arteriosclerosis.”
A special stamping technique makes examination possible
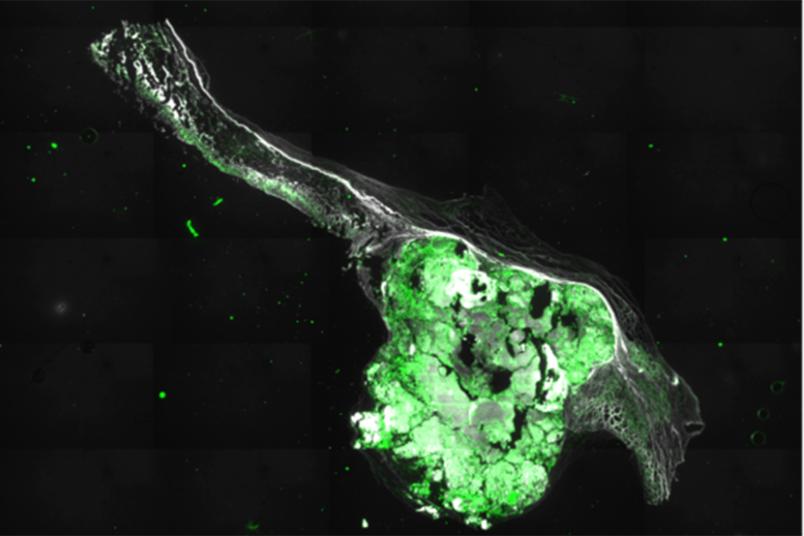
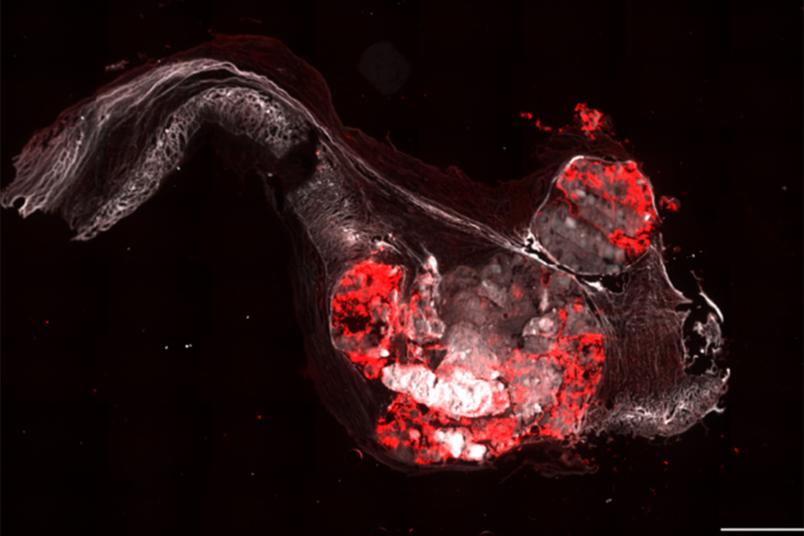
The research team developed a special technique to isolate the endothelial cells for examination. They placed a defective human aortic valve, which has been removed during surgery, between two glass slides and pressed a frozen stamp onto it. This caused the endothelial cells to freeze to the glass slides, with the cells facing the heart on one slide, and those on the aortic side on the other. The scientists can now examine the thin layer of cells in detail.
By staining the cells, they can determine how permeable the endothelium is. The more permeable it is to proteins from the blood, the more diseased the endothelium is. They also examined the RNA produced by the cells. This allows the researchers to determine which genes are currently being expressed.
Calcification is only one-sided if there are three leaflets
“In people whose aortic valve consists of three leaflets – which is usually the case – you can see with the naked eye that calcification occurs primarily on the aortic side of the valve, not so much on the cardiac side,” explains Adrian Brandtner, doctoral student and lead author of the study. The staining analysis and RNA sequencing showed that on the aortic side, the endothelium was more permeable and expressed more genes that indicate calcification processes. Thus, the endothelium is clearly involved in the disease.
“But it was also interesting to note that this is quite different in people whose aortic valve consists of only two leaflets,” says the researcher. People with this genetic predisposition tend to suffer from aortic valve stenosis earlier in life. In these individuals, the endothelium on both sides of the valve is equally damaged, meaning it is permeable and affected by calcification. “Aortic valve stenosis in people with two leaflets is therefore clearly a very different disease than in people with three leaflets,” concludes Brandtner.
The researchers hope their findings will contribute to a deeper understanding of the development of aortic valve stenosis. “It would be great if, one day, we could use medication to intervene when stenosis is just beginning and halt the progression of the disease,” Wenzel hopes.