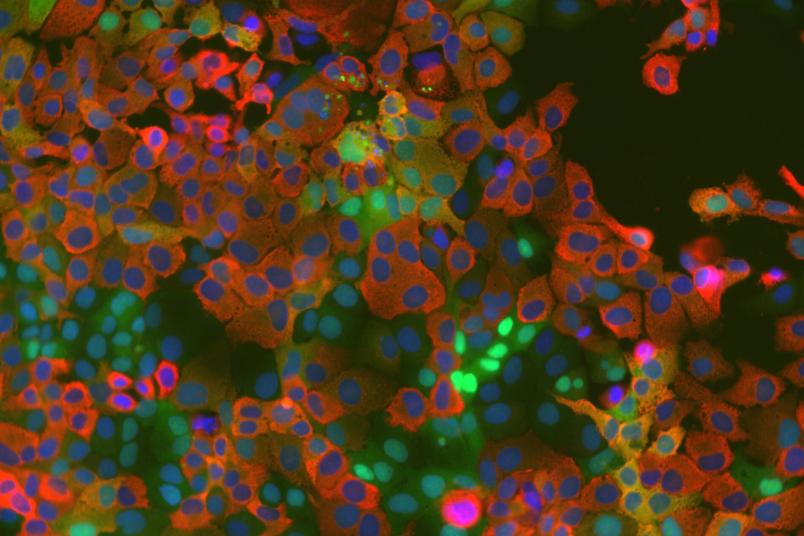
Cell culture cells expressing a kinase activity reporter (green) after infection with the human coronavirus HCoV-229E (red). Cell nuclei are stained blue.
Virology
Newly Discovered Host Mechanism in Coronaviruses
The discovery could serve as a starting point for antiviral strategies.
A research team at Ruhr University Bochum, Germany, has identified a previously unknown cellular mechanism crucial to the replication of coronaviruses: c-Jun N-terminal kinase (JNK) is activated during infection with human coronavirus HCoV-229E and mediates the phosphorylation of the viral nucleocapsid (N) protein, an integral step in the virus cycle. These results aid in better understanding virus-host interactions and may open new approaches to exploring antiviral strategies in the long term. The team led by Dr. Yannick Brüggemann and Professor Eike Steinmann reports its findings in the journal npj Viruses from September 18, 2025.
Virus production decreases when the kinase is blocked
By using live-cell microscopy, quantitative immunofluorescence, and biochemical analyses, the researchers were able to demonstrate that JNK is specifically activated in infected cells. They used a kinase translocation reporter (KTR) to directly visualize the marked increase in JNK activity approximately 16 hours after infection. If the kinase is blocked by specific inhibitors, virus production was reduced significantly. This is the case for both HcoV-229E and SARS-CoV-2.
In cooperation with the group led by Professor Michael Kracht at University of Giessen, the team was also able to show that JNK phosphorylates specific serine residues on the N protein. These sites are conserved in various coronaviruses, indicating that JNK plays a joint role in the replication of various virus types. “Our data highlight JNK as an important host factor that is directly involved in the modification of the N protein, a critical step in virus replication,” explains Brüggemann. “The fact that inhibition of JNK hampers the replication of both HCoV-229E and SARS-CoV-2 shows the potential of this signal pathway as a future starting point for new antiviral agents,” adds Steinmann.